
Is HBr an Acid or Base? Techiescientist
For instance, the base-induced elimination of HBr from 1-bromo-2-phenylethane proceeds 7.11 times faster than the corresponding elimination of DBr from 1-bromo-2, 2-dideuterio-2-phenylethane. This result tells us that the C-H (or C-D) bond is broken in the rate-limiting step, consistent with our picture of the E2 reaction as a one-step process.

The mechanism of HBrelimination and dihaloelimination of HBCDs. The
Certain ethers are cleaved with HBr. It also catalyzes alkylation reactions and the extraction of certain ores. Significant industrial organic compounds prepared from hydrobromic acid include allyl bromide, tetrabromobis (phenol), and bromoacetic acid.. (146)) < 55JA3465 >; however an excess of FeCl 3 causes elimination and halogen exchange.

PPT Elimination Reactions PowerPoint Presentation, free download ID
5. By definition, an E1 reaction is a Unimolecular Elimination reaction. This means the only rate determining step is that of the dissociation of the leaving group to form a carbocation. Since E2 is bimolecular and the nucleophilic attack is part of the rate determining step, a weak base/nucleophile disfavors it and ultimately allows E1 to.

(4) Which of the following shows a mechanism of a concerted elimination
In recent studies, Ohgiya, Nishiyama, et al. and we have explored the DBU-promoted regioselective HBr-elimination of vicinal dibromides having an adjacent O-functional group. 3(g), 6 A typical example is illustrated in Scheme 1, which shows that both the elimination reactivity and regioselectivity are affected by the presence or absence of the neighboring O-functional group in the substrate.
PPT AQA organic reaction mechanisms PowerPoint Presentation, free
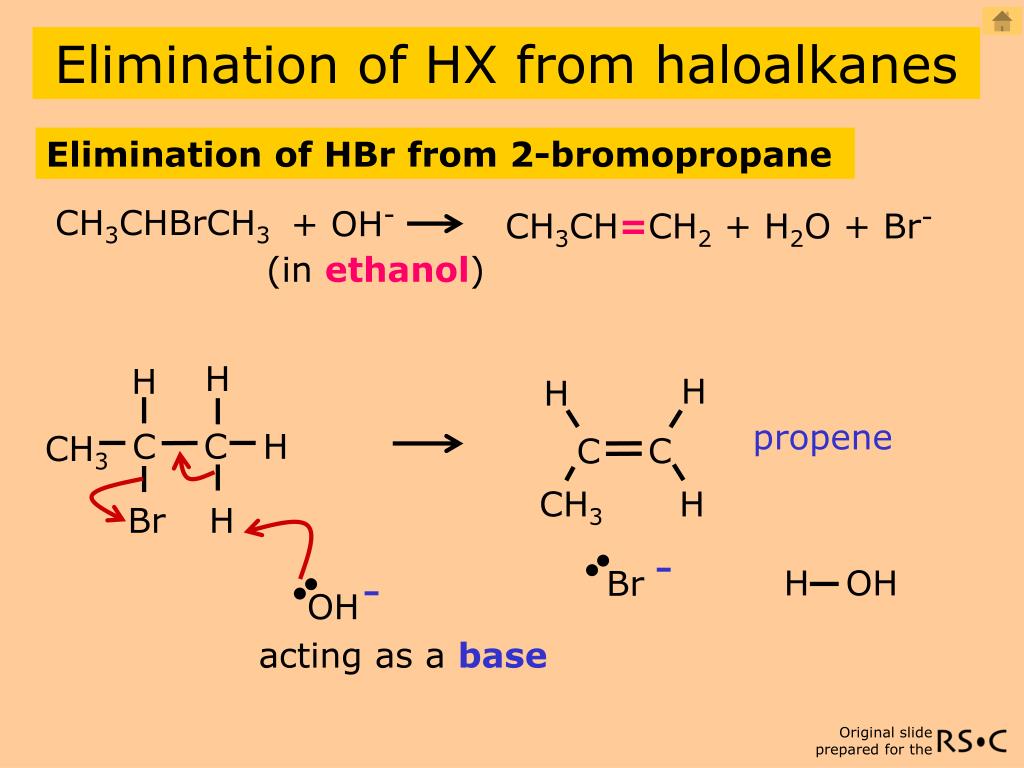
elimination reaction: R2CH-CBrR2 + NaOH ==> R2C=CR2 + H2O + NaBr as well as/versus the substitution reaction: R2CH-CBrR2 + NaOH ==> R2CH-C (OH)R2 + NaBr In both cases R = H, alkyl or aryl and 'HBr' is eliminated or 'Br' substituted.

The E2 Reaction Mechanism
Chemical activation experiments and computational methods have been used to study the unimolecular reactions of C2H5CH2Br and C2D5CHFBr with 90 and 93 kcal mol-1 of vibrational energy, respectively. The four-centered elimination reactions of HBr and DBr are the dominant reactions; however, 2,1-DF, 1,1-HBr, and 1,1-HF reactions are also observed from C2D5CHFBr. The main focus was to search.

PPT Elimination Reactions PowerPoint Presentation, free download ID
E1 elimination - An overview. The reverse of electrophilic addition is called E1 elimination. We will begin by looking at some non-biochemical E1 reactions, as the E1 mechanisms is actually somewhat unusual in biochemical pathways. E1 elimination: An E1 elimination begins with the departure of a leaving group (designated 'X' in the general.

20.3.2 Describe/explain the mechanism for elimination of HBr from
In the other (bottom) pathway, methoxide ion acts as a base (rather than as a nucleophile) in an elimination reaction. As we will soon see, the mechanim of this reaction is single-step, and is referred to as the E2 mechanism. In the methanol solvent used here, methanethiolate has greater nucleophilicity than methoxide by a factor of 100.

Intrinsic reaction coordinate energies for HBr elimination via 3centre
Chapter First Online: 04 January 2022 953 Accesses Abstract Elimination reactions are the reverse of addition reactions. These involve elimination of two or four groups attached to adjacent carbon atoms in a substrate forming a multiple bond. Download chapter PDF 1 Introduction Elimination reactions are the reverse of addition reactions.

Solved 6. The observed rate law for the HBr elimination of
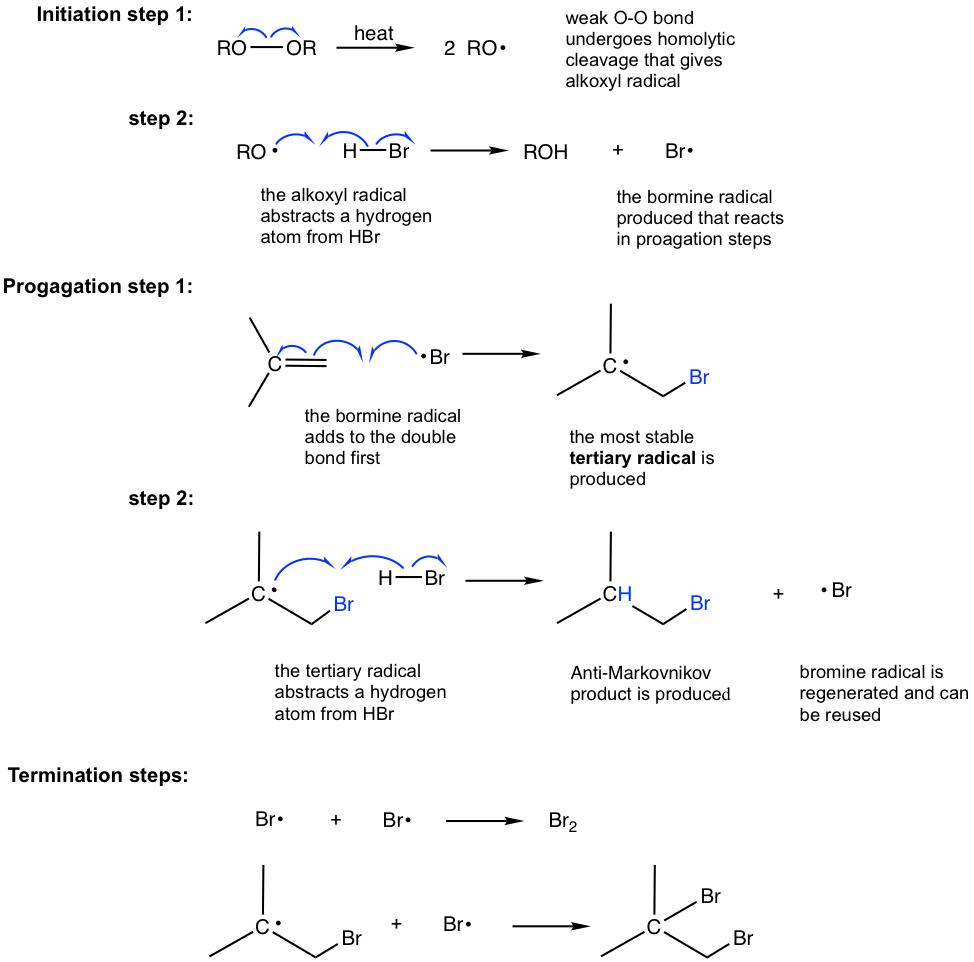
1. Free Radical Addition Of HBr To Alkenes Leads To "Anti-Markovnikov" Products As discussed previously, alkenes normally react with HBr to give products of "Markovnikov" addition; the bromine ends up on the most substituted carbon of the alkene, and the hydrogen ends up on the least substituted carbon.
Carbonyl Mechanisms Elimination (1,2Elimination)
One of the first proposals for the mechanism of the E2 reaction. Prof. Ingold mentions in this paper, " It follows from the basic hypothesis that the ease of removal of the b-proton (reaction A) depends (a) on its vulnerability, (b) on the proton-avidity of the attacking anion". Influence of poles and polar linkings on the course pursued by.

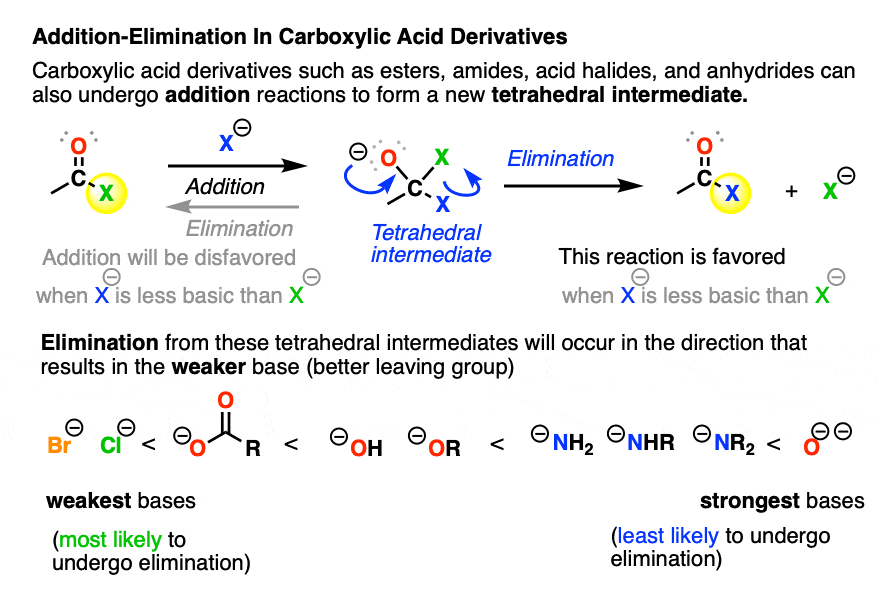
Simply Mechanisms 7d. Nucleophilic Addition Elimination (Ethanoyl
It could be understood as that with the presence of excess base (OH-) in the reaction mixture, HBr reacts with OH- to give H2O and Br-. The following discussion of the mechanism will help you to understand this better.

Halogenoalkanes Elimination Reactions (ALevel) ChemistryStudent
Suggest a mechanism to explain the formation of (chloromethyl)benzene from methylbenzene. Strategy Draw out a balanced chemical equation for this reaction, and work out what the driving force for this process is. This reaction is more than likely a radical process as it requires UV radiation.
10.2 Reactions of Alkenes Addition of Hydrogen Halide to Alkenes
Master Organic Chemistry Reaction Guide Addition of HBr to Alkenes Description: Treatment of alkenes with hydrobromic acid will result in the formation of alkyl bromides. Notes: This is an addition reaction. Note that the bromine always ends up at the more substituted carbon of the alkene (Markovnikoff-selectivity) Examples:

Intrinsic reaction coordinate energies for HBr elimination via 3centre
Elimination Reactions. E2 and E1 Elimination of Cyclohexane Derivatives. In this post, we will talk about the E2 and E1 elimination reactions of substituted cyclohexanes. Let's start with the E2 mechanism. When the following substituted cyclohexane is treated with sodium ethoxide, an E2 elimination is expected to occur as we have a strong.

Alkene + HBR + ROOR Reaction Mechanism YouTube
Elimination Reactions 1. The E2 Reaction. We have not yet considered the factors that influence elimination reactions, such as example 3 in the group presented at the beginning of this section. (3) (CH 3) 3 C-Br + CN (-) ——> (CH 3) 2 C=CH 2 + Br (-) + HCN We know that t-butyl bromide is not expected to react by an S N 2 mechanism. . Furthermore, the ethanol solvent is not sufficiently.